401. Synthetic laboratory
Head: Zoltán Bánóczi, Ph.D.
Address: Budapest, Pázmány P. sétány 1/A, 401.
Telefon: 06-1-372-2500
Fax: 06-1-372-2620
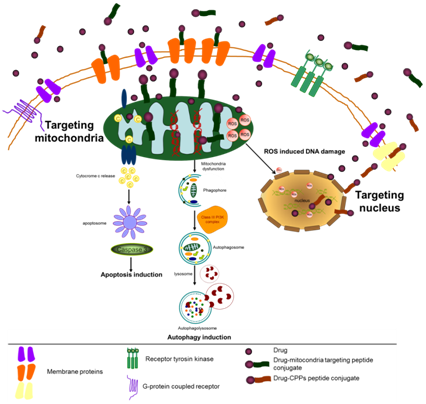
The main research topic in our laboratory is the synthesis and biological evaluation of bioconjugates in order to prepare new chemotherapeutically applicable peptide based compounds. Drug conjugation to peptide carrier moiety is used to increase the biological properties of the drugs (e.g. increase the cellular uptake, decrease the side effects, prevention of multidrug resistance, increase the water solubility, etc). On the other hand, proapoptotic peptides are conjugated to peptide carriers to enhance the apoptotic processes in the cancer cells.
Among the drug molecules, antracyclins (daunorubicin, doxorubicin), vinca-alkaloids and folic acid antagonists (methotrexate, pemetrexed) are used for conjugations. The conjugates are built up by different coupling methods (amide, squaric amides, oxime, hydrazone, thioether). The antracyclin containing conjugates can be used without any modification by own fluorescence property not only in the in vitro cytostatic assays but also in the cellular uptake studies. In the case of the other drugs without fluorescence feature; fluorescence labelling is needed for the evaluation of their cellular uptake assays.
The prepared bioconjugates will be applied in the area of research that belongs to the cell penetrating peptides (CPPs). Drug molecules are conjugated to these peptides, because they can pass through the cell membranes, and they can deliver the drugs into the cytosol/nucleus. Among the CPPs penetratin and oligoarginine are mostly used in our research. We also make an attempt to synthetize new, short cell penetrating peptides to minimally decrease the interaction between the drug and the peptide.
Peptides are prepared manually by solid phase peptide synthesis using Boc/Bzl or Fmoc/tBu strategy. We have also chance to apply automatic peptide synthetizer (Syro) for the synthesis. Teflon HF apparatus (409. laboratory) is available for detachment of the peptides from the resin in case of Boc/Bzl strategy. The conjugations are mainly carried out in solution phase using different chemical ligation methods. The crude peptides or bioconjugates are purified by RP-HPLC or coloumn chromatography (414. laboratory).
References:
Hudecz F, Banoczi Z, Csik G.: Medium-sized peptides as built in carriers for biologically active compounds. Med Res Rev. 25, 679-736 (2005).
Bánóczi, Z., Tantos, A., Farkas, A., Tompa, P., Friedrich, P., Hudecz, F. Synthesis of cell-penetrating conjugates of calpain activator peptides. Bioconjugate Chem., 18, 130-137 (2007).
Bánóczi, Z., Alexa, A., Farkas, A., Friedrich, P., Hudecz, F. Novel cell-penetrating calpain substrate. Bioconjugate Chem., 19, 1375-1381 (2008)
Szabo, I; Orban, E; Schlosser, G; Hudecz, F; Banoczi, Z Cell-penetrating conjugates of pentaglutamylated methotrexate as potential anticancer drugs against resistant tumor cells. Eur J Med Chem. 115, 361-368 (2016)
Ba´nóczi Z; Gorka-Kereskényi A; Reme´nyi J; Orbán E; Hazai L; Tőkési N; Oláh J; Ovádi J; Béni Z; Háda V; Szántay Cs; Hudecz F; Kalaus G; Szántay Cs: Synthesis and in vitro antitumor effect of vinblastine derivative-oligoarginine conjugates. Bioconjugate Chem., 21, 1948-1955 (2010)
Bánóczi Z; Peregi B; Orbán E; Szabó R; Hudecz F: Synthesis of Daunomycin - oligoarginine conjugates and their effect on Human leukemia cells (HL-60). Arkivoc, 2008, Part (III), 140-153 (2007)
Among the drug molecules, antracyclins (daunorubicin, doxorubicin), vinca-alkaloids and folic acid antagonists (methotrexate, pemetrexed) are used for conjugations. The conjugates are built up by different coupling methods (amide, squaric amides, oxime, hydrazone, thioether). The antracyclin containing conjugates can be used without any modification by own fluorescence property not only in the in vitro cytostatic assays but also in the cellular uptake studies. In the case of the other drugs without fluorescence feature; fluorescence labelling is needed for the evaluation of their cellular uptake assays.
The prepared bioconjugates will be applied in the area of research that belongs to the cell penetrating peptides (CPPs). Drug molecules are conjugated to these peptides, because they can pass through the cell membranes, and they can deliver the drugs into the cytosol/nucleus. Among the CPPs penetratin and oligoarginine are mostly used in our research. We also make an attempt to synthetize new, short cell penetrating peptides to minimally decrease the interaction between the drug and the peptide.

Peptides are prepared manually by solid phase peptide synthesis using Boc/Bzl or Fmoc/tBu strategy. We have also chance to apply automatic peptide synthetizer (Syro) for the synthesis. Teflon HF apparatus (409. laboratory) is available for detachment of the peptides from the resin in case of Boc/Bzl strategy. The conjugations are mainly carried out in solution phase using different chemical ligation methods. The crude peptides or bioconjugates are purified by RP-HPLC or coloumn chromatography (414. laboratory).
